Shop By Collections
Falsified Certificates of Analysis
It was bound to happen, but we had our first ever incident of TrustPointe COAs...
Read more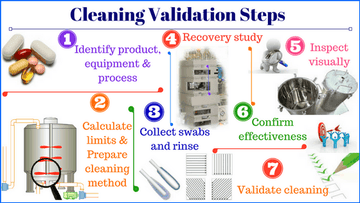
Importance of Cleaning Validation in Preventing Cross-Contamination
Let’s get the boring part out of the way first, then we can get to...
Read more
Laboratory Error & Investigations
It is hard to believe, but every now and then people make mistakes. Analysts might...
Read more
Sampling & Testing
Last update: 25-Oct-2024. Peptide users should consider the effect of sample size on understanding overall...
Read more