USP <61> Nonsterile Microbial Enumeration
United States Pharmacopeia (USP) <61> microbial enumeration (counting) for non-sterile dosage forms (tablets, liquids, etc). TAMC/TYMC results reported on a COA.
Lead time is 10 - 14 days for incubation and analysis.
Please read and agree to our Terms of Service before placing your order.

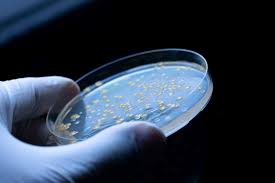
USP <61> Nonsterile Microbial Enumeration
What People Are Saying
Discover more in our FAQ
What types of samples do you test?
We test a wide range of products including capsules, tablets, powders, liquids, and peptides for potency, sterility, and more.
How long does testing take?
Standard turnaround is 3–5 business days. Rush options are available upon request.
What information should I include with my sample?
Please include the label claim (mg), sample identifier (e.g. cap color or lot number), client name, and tracking number.
Will I receive a Certificate of Analysis (COA)?
Yes, every completed test includes a detailed, lab-verified COA tailored to your product and compliance requirements. COAs are delivered electronically to the email provided at checkout.











